validity of cbc results|(PDF) Verification and Standardization of Blood Cell : Pilipinas According to the results of our study, CBC parameters are more likely to change in temperate climate, and caution should be taken to store them at 4°C when a .
The 2024 World Series of Poker may still be six months away, but it is never too early to start planning for the next Main Event. This year, WSOP US has surprised players across all four regulated sites with a special freeroll on January 31. The freeroll will award the winner with a seat in the 2024 WSOP Main Event, and all existing and new .
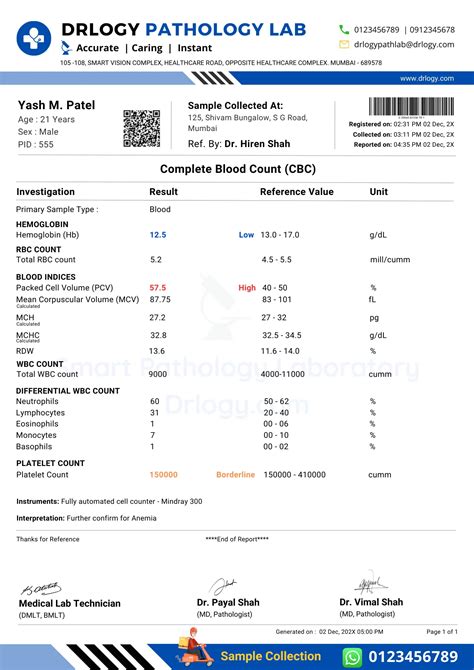
validity of cbc results,Specimens stored > 12 h. for CMP may generate unreliable results. For CBC, samples could reliably be stored for 24 h. For longer storage, refrigeration (at 4 °C) would be a better choice.Fig. S7 - How Long can we Store Blood Samples: A Systematic Review and .Fig. S17 - How Long can we Store Blood Samples: A Systematic Review and .Fig. S22 - How Long can we Store Blood Samples: A Systematic Review and .
Fig. S28 - How Long can we Store Blood Samples: A Systematic Review and .validity of cbc results According to the results of our study, CBC parameters are more likely to change in temperate climate, and caution should be taken to store them at 4°C when a .

In a verification report, the results for imprecision (repeatability and between batch) should be reported including the mean and CV (%). The state-of-the-art CV (%) for imprecision (reproducibility . In a verification report, the results for imprecision (repeatability and between batch) should be reported including the mean and CV (%). The state-of-the-art CV (%) for imprecision (reproducibility .
The aim of this study is to assess the comparability of CBC parameters between capillary and venous samples, and to extend previous research by examining . The complete blood count (CBC) is one of the most commonly requested clinical laboratory tests. It provides important information on blood cell numbers, .A complete blood cell count (CBC) is one of the most common laboratory tests in medicine. For example, at our institution alone, approximately 1800 CBCs are ordered every day, and 10% to 20% of results are .
validity of cbc results (PDF) Verification and Standardization of Blood Cell Automated hematology analyzers generate accurate complete blood counts (CBC) results on nearly all specimens. However, every laboratory encounters, at times, some . Verification and Standardization of Blood Cell Counters for Routine Clinical Laboratory Tests. March 2015. Clinics in Laboratory Medicine 35 (1):183-196. DOI: 10.1016/j.cll.2014.10.008. Source..Values & Chart. 3 Major Components. Uses of the CBC Test. Guide. What Is a Complete Blood Count (CBC) Test? A complete blood count (CBC) is a common blood test. The complete blood count (CBC) test is .Precision. The laboratory must verify short-term (between run) and long-term (between day) reproducibility of the device. 1 Short-term reproducibility should be verified using normal .
The complete blood cell count (CBC) is a crucial test for the diagnosis and management of a variety of haematological disturbances, provided that the quality throughout the testing process can be guaranteed. . Several articles have been published about the stability of whole blood specimens for CBC testing, but results are often .(PDF) Verification and Standardization of Blood Cell Results. Among CBC parameters, white blood cell, red blood cell, hemoglobin, mean cell hemoglobin (MCH), neutrophils and lymphocytes were stable at all three temperatures up to 48 hr. Monocytes, eosinophils, MCH concentration, hematocrit (Htc), and red cell distribution width‐coefficient of variation showed statistically significant . The complete blood count (CBC) with differential leukocyte count (DLC) is one of the most common tests requested by physicians. The results of this test are affected by storage temperature and time of incubation. This study was designed to evaluate the stability of hematologic parameters in blood specimens stored for 48 h at three .The full blood count test is used in the preoperative setting to detect anaemia, bleeding disorders, inherited and acquired haematological disorders, and the effects of other systemic diseases. The results may be used to plan the use of blood products and blood salvage techniques in the perioperative period. The test is considered safe and is . Reliability is about the consistency of a measure, and validity is about the accuracy of a measure.opt. It’s important to consider reliability and validity when you are creating your research design, planning your methods, and writing up your results, especially in quantitative research. Failing to do so can lead to several types of research .
Finally, middleware rules achieved very high autoverification rates of 97.2% and 88.3% for CBC and CBCD results, respectively. . Six hundred seventeen rules were created according to the proposed model. 1,976 simulation results were created for validation. Our results showed that manual review limits are the most critical step in . Results. Of 631 PCPs who received an invitation to participate in the survey, 356 (56.4%) responded and 31 (4.9%) were excluded, for an adjusted eligible sample size of 600, yielding 325 completed surveys (response rate, 54.2%). Of the 325 participants who completed surveys, 180 (55.4%) were men; age of participants was not assessed.

Their results therefore do not reflect daily practice. In this study the validity of urine tests for UTI is deterined under daily practice conditions, without the use of a protocol. The results show a validity considerably lower than under optimal conditions. Specificity in particular was lower, even for simple tests like the nitrite reaction.
validity of cbc results|(PDF) Verification and Standardization of Blood Cell
PH0 · Verification and quality control of routine hematology
PH1 · Validation of Hematology Analyzers : October 2016
PH2 · Unreliable Automated Complete Blood Count Results: Causes,
PH3 · Stability and comparison of complete blood count parameters between
PH4 · Stability and comparison of complete blood count parameters
PH5 · Reliability of Parameters of Complete Blood Count With Different
PH6 · Obtaining Reliable CBC Results in Clinical Laboratories
PH7 · How to Interpret and Pursue an Abnormal Complete Blood Cell Count in
PH8 · How to Interpret and Pursue an Abnormal Complete
PH9 · How Long can we Store Blood Samples: A Systematic Review
PH10 · Complete Blood Count (CBC) Test and Results
PH11 · (PDF) Verification and Standardization of Blood Cell